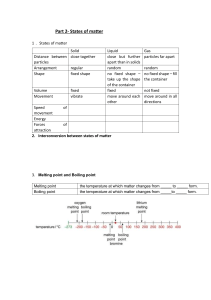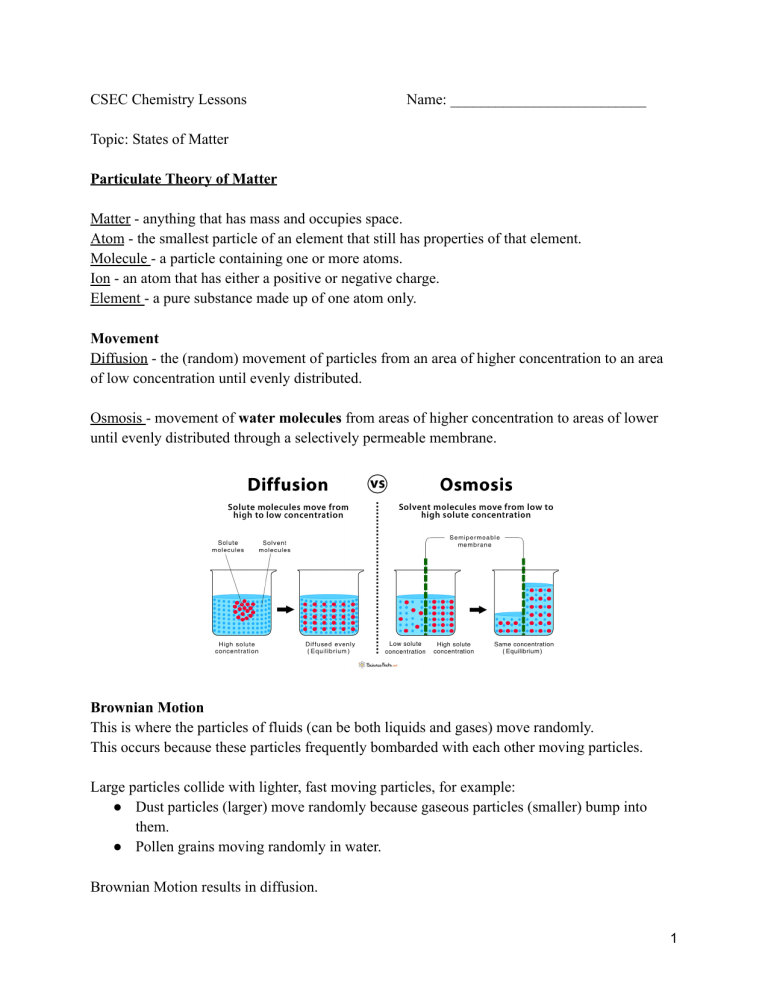
CSEC Chemistry Lessons Name: __________________________ Topic: States of Matter Particulate Theory of Matter Matter - anything that has mass and occupies space. Atom - the smallest particle of an element that still has properties of that element. Molecule - a particle containing one or more atoms. Ion - an atom that has either a positive or negative charge. Element - a pure substance made up of one atom only. Movement Diffusion - the (random) movement of particles from an area of higher concentration to an area of low concentration until evenly distributed. Osmosis - movement of water molecules from areas of higher concentration to areas of lower until evenly distributed through a selectively permeable membrane. Brownian Motion This is where the particles of fluids (can be both liquids and gases) move randomly. This occurs because these particles frequently bombarded with each other moving particles. Large particles collide with lighter, fast moving particles, for example: ● Dust particles (larger) move randomly because gaseous particles (smaller) bump into them. ● Pollen grains moving randomly in water. Brownian Motion results in diffusion. 1 The Kinetic Theory of Matter States that matter is made up of tiny particles that are in constant motion due to the kinetic energy they contain. Characteristic Solid Liquid Gas Regular pattern Irregular pattern Irregular pattern Closeness of particles Very close / touching Close Far apart Kinetic energy Low - particles vibrate around a fixed point Moderate - particles move from place to place High - particles move freely and quickly Arrangement of paticles Physical Properties ● Shape ● Volume ● Compressibility: solids & liquids cannot, gases can - room to force particles closer ● Density: high → low; more particles in a space to less ● Melting/ boiling point: high → low; energy needed to break/form bonds decreases from solid to gas Physical Property Solid Liquid Gas Shape Volume Compressibility Melting/Boiling point Density (amount of particles in a given volume) 2 Changes of State Melting : solid to liquid (s→l) Freezing: liquid to solid (l→s) Boiling: liquid to gas (l→g) Condensing: gas to liquid (g→l) NB. Sublimation: transition directly from solid to gas without passing through liquid state (or vice versa) Eg. iodine - solid I2 sublimes on heating to give purple vapour & condenses to form crystals Dry ice - solid carbon dioxide give white fumes @ r.t.p Mothballs/ air fresheners ~ Boiling point - the temperature @ which the vapour pressure of a liquid equals 1 atm ~ Freezing/ melting point - the temperature @ which a liquid and its solid are at equilibrium. At temperatures: - Below mtp substance exist as solid only. - Above mtp but below btp substance is liquid only - Above btp substance is gas only - At mtp only substance exist as solid and liquid (2 states) - At btp only substance exist as liquid and gas (2 states) Practice: Substance X melts at -114oC and boils at -85oC. Draw a number line, put in mpt and bpt, label the 3 states and place 0oC in the correct place relative to mpt/ bpt. 3 Heating Curves 1. Solid is being heated. Temperature gradually increases so kinetic energy increases while potential energy remains constant. 2. Solid melts (state change). When melting point is reached the temperature stops increasing and remains constant because the heat energy is used to weaken bonds to change from solid to liquid. Kinetic energy constant, potential energy increases. 3. Liquid heated. When all solid melts, temperature starts to rise again. Kinetic energy increases, potential energy constant. 4. Liquid boiling. Temperature remains constant as heat energy is used to break bonds between particles to convert to gas. Kinetic energy constant, potential energy increases. 5. Gas heated. Temperature increases again and will continue to do so as long as heat is being applied. NB: Plateaus - changes of states, energy weakens bonds so temp and KE constant, PE increases. Slopes - heating of states, temp increase so KE increases, PE constant Kinetic energy - the energy an object/ particle possesses due to its motion Potential energy - the stored energy in any object or system by virtue of its position or arrangement of parts. 4 Cooling Curves Starts as a gas goes to solid. Eg. water vapour to ice Temperature decreases so gas is cooled. KE decreases, PE constant Gas condenses. Temp. remains constant. Particles move closer together and bonds strengthen. KE constant, PE decreases. Liquid cooled. Temp. decreases. Particles move slower because KE decreases, PE constant. Liquid freezes. Temp constant. Particles move even closer and form stronger bonds. KE constant, PE decreases. Solid cooled. Temp decreases until it is no longer being cooled. KE decreases, PE constant. Practice: 1. Acetone boils/condenses at 56oC and melts/freezes at -95oC. 2. Ethanol has a melting point of -117.3oC and a boiling point of 78.5oC 5